Get it? Because it’s a thread about iron, but you also have threads literally made of… never mind.
What I want to discuss here is Thrive’s iron oxidation metabolism. A very real source of energy, represented in Thrive by the Rusticyanin protein and the Ferroplast organelle. We’ll discuss how this energy source works in Thrive, and how well this corresponds to (our scientific understanding of) reality.
Skip to the end for a summary of recommendations and suggestions.
The organelles
To start with, while “Rusticyanin” might sound like a video game designer came up with it, it’s a real thing, and is strongly involved in exactly what Thrive uses it for. Meanwhile the “Ferroplast” is, hilariously, a real company that sells pipes. In all seriousness, the ferroplast is a very reasonable hypothetical organelle made of an iron oxidising bacterium, or similar. It’s good speculative evolution, I only wonder if it shouldn’t be disabled by the LAWK switch, since I’m not aware of any eukaryote having such a structure.
So far, mostly good!
The chemistry
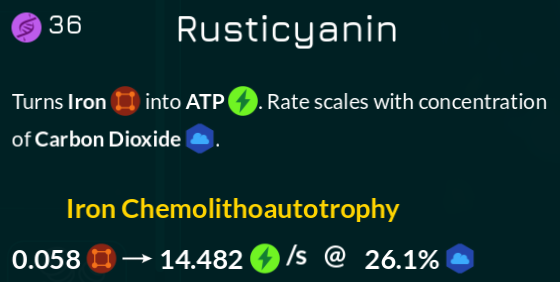
Ah, my own field of expertise. First let’s look at Thrive again, straight from the hypothetical horse-equivalent’s mouth:
I won’t discuss the Ferroplast separately from here since it’s the same process. In chemical reaction notation, I’ll write that down as:
Iron + CO2 → ATP
It should also be noted that unlike some other compounds, iron only spawns as chunks, not as clouds directly.
Let’s now look at the real world. Wikipedia has a nice page, but here’s also a (freely available!) review paper.
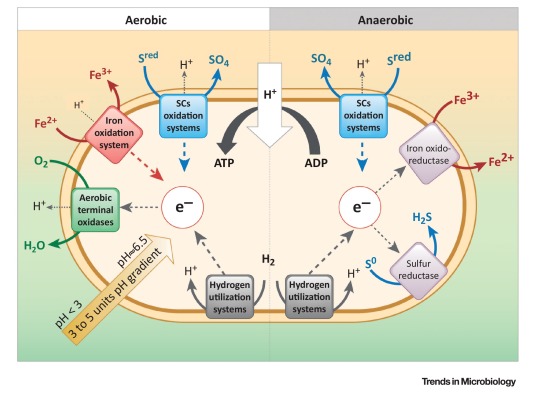
The first thing to note is that “iron” is not just iron. Iron in nature primarily exists in two forms: ferrous/Fe(2+)/Fe(III) and ferric/Fe(3+)/Fe(III). Your favourite metallic iron spork is plain Fe, but not directly relevant here. While Fe(II) dissolves in water relatively well, Fe(III) would very much like not to be dissolved in water. Fe(III) therefore rapidly forms minerals (rocks) that we know as rust or iron ore. Iron oxidising bacteria turn Fe(II) into Fe(III) to gain energy. Thrive’s “Iron” is thus Fe(II).
Since our Thrive organisms are actually converting soluble Fe(II) into insoluble Fe(III), you might then argue that instead of going after iron chunks, we should be going after plain iron clouds and poop rocks. Personally I think what Thrive does here is fine. Fe(II) solubility is still not great, so it is reasonable to show some Fe(II) minerals slowly dissolving, or even some lost metallic Fe reacting with the water to form Fe(II). You could equally expect to see a bunch of spawned Iron compound clouds though. Meanwhile, waste product Fe(III) rocks are not further used by Thrive, so would be a pointless addition.
There is also not just one way to do this. As it turns out, there are more or less 4 categories recognised. With somewhat simplified reactions, I will list them here:
Aerobic iron oxidation:
Using oxygen to oxidise Fe(II) for direct energy:
Fe(II) + O2 + H → Fe(III) + H2O + ATP
Or, in Thrive terms and leaving out everything Thrive does not simulate:
Iron + Oxygen → ATP
Anaerobic iron oxidation:
Oxidising Fe(II) for direct energy using something that’s not oxygen:
Fe(II) + NO3 + H2O → Fe(III) + NO2 + H + ATP
Or:
Iron → ATP
I just showed nitrate as an example here, but there are actually a ton of options.
Iron-based chemolithoautotrophy (or chemosynthesis)
Using oxygen to oxidise Fe(II), in order to produce biomass from CO2:
Fe(II) + O2 + CO2 + H2O → [CH2O] + H + H2O
Or:
Iron + Oxygen + CO2 → Glucose
For complicated chemistry reasons, this one only really works in very acidic water. But Thrive does not model acidity anyway, so who cares!
Photoferrotrophy
Using light to oxidise Fe(II), in order to produce biomass from CO2:
Fe(II) + HCO3 + H2O + light → [CH2O] + H
Or:
Iron + CO2 + light → Glucose
Basically an alternative form of photosynthesis that does not produce oxygen.
Have you spotted the problem yet? Thrive’s current Iron consuming organelles don’t match any of these! The anaerobic (without oxygen) ATP production comes perhaps the closest, but the only reason to add CO2 as a requirement would be if it was producing glucose!
The description
One more quick point here on the description of the organelles:

As I mentioned a few times above, converting Fe(II) into Fe(III) for energy is called iron oxidation. Iron respiration is, in a way, the opposite: using Fe(III) instead of for example oxygen to oxidise something else. Thrive obviously does not include this type of reaction, but that does not mean respiration and oxidation are interchangeable words.
In addition, the “process” is named “Iron Chemolithoautotrophy”. As can be seen in my overview of the chemistry, this is also inaccurate. You can only speak of autotrophy when an organism makes organic compounds (glucose) out of non-organic carbon (CO2). Since no glucose is produced, a different name should be used.
Beta/experimental features: Siderophores
So, many players are probably not aware of this but by turning on “experimental features” you can enable a prototype for a proposed alteration to how iron consumption works.
In the new mechanics, rusticyanin lets you build up a compound (apparently planned to be named siderophores), that can be shot out of your cell to break small iron chunks off of large Iron chunks. If implemented, large iron chunks would probably give off iron compound clouds less quickly or not at all.
Now let’s take a look at reality. Siderophores are real, and they do help organisms absorb iron from insoluble chunks. However, as I stated earlier in this post, not all iron is the same. Siderophores are not used to aid in energy production, they are used to break down Fe(III)-based minerals (Ferric) so that the iron can be used as nutrients (like phosphate and ammonia in Thrive). So, obviously the name is wrong.
But more importantly the concept is unfortunately wrong. Like I said before, unlike Fe(III) minerals, Fe(II) (ferrous) minerals dissolve well enough in water. So the Fe(II) that iron-oxidising bacteria need dissolves out of rocks in sufficient numbers on its own, making a game design where this is a specific problem that must be overcome is misleading.
From a pure scientific accuracy point of view: this change would make the game less accurate.
Recommendations
My perspective here is purely to give recommendations based on scientific accuracy, I am not much of a game developer or programmer. I did try to construct my more firm recommendations in a way that seems like the least work.
Recommendations
- Remove CO2 scaling from rusticyanin and ferroplasts.
- Limit ferroplasts to non-LAWK. Edit: apparently currently a mechanical limitation?
- Rename the process of these organelles from “Iron Chemolithoautotrophy” to simply “iron oxidation”.
- Correct the description of these organelles to replace “iron respiration” with “iron oxidation”
Suggestions
Fun to have, but not at all necessary/something a volunteer could pick up if interested someday:
- An organelle/modification of the existing organelles to switch to oxygen-dependent iron oxidation, with higher efficiency.
- An organelle/modification to perform actual chemolithoautotrophy, requiring iron, oxygen and CO2 to produce glucose.
- An organelle/modification for photoferrotrophy, requiring iron, light and CO2 to produce glucose.
Things that would be accurate but are probably a bad idea
- let iron oxidising bacteria poop rocks.
- Add a way for cells to use rocks instead of oxygen.
And if you actually made it this far, thanks for reading!