I thought to ask this in the future game section, but I want to know the chemistry behind this first.
Cells can produce ammonia thanks to the readily available nitrogen in the atmosphere, however, there is no analogue “phospate production” protein or organelle, nor plans to add it. In theory this is due to the relative rarity of phosphorus, so it makes sense.
But then I realized ATP stands for Adenosine TRIPHOSPHATE, so what exactly is preventing a cell from expending energy to break apart a portion of its ATP stores to salvage the phosphates?
I’m not a theorist, but from what I remember the problem with that approach is that then you are stuck with ADP and you must use phosphate to get back to ATP.
So by stealing the phosphorus from ATP, you permanently reduce the amount of energy the cell can work with, and eventually just die completely as no ATP can be recharged from ADP and as a result no biological processes can keep running that use energy from ATP.
Phosphorus exists as phosphate in nature. You get it wherever you find it, and its usable. There is no phosphorus fixing.
I am not a theorist of the team, but I do have academic knowledge about that subject (literally learning life science right now, More than meets the requirements, as I am graduating this year with a bachelor’s degree) and I want to add more than what I added here:
He is right, but not absolute. Phosphate is a very important component in the creation of energy, since ATP is the main one that developed in evolution by chance, but not only as energy (there are others that can be used such as TTP, GTP and CTP since they are also used not only as a source of energy, but this is already a deviation from the topic) these also as a source Phosphate for many biological processes, in a process called “phosphorylation” (this can be said as if to give energy by giving his phosphate to a protein in order to activate it or change its structure for its function), but technically “stealing phosphate” from ATP already exists in all the cells we know of and this is done for almost every known biological process which involves ATP during its conversion to ADP (this is how DNA, RNA, and many other things are created that I will not detail here)
The problem with phosphate is how much it is actually present in nature.
Let’s start with the basics. Phosphorus, which is a key element of life. It is one of the six most common in any living body - hydrogen, carbon, oxygen, nitrogen, sulfur and phosphorus. But compared to the other 5, phosphorus is the only atom in nature that is not present in any form other than solid, and usually, exists bonded to other atoms, commonly phosphate.
So you can say - phosphorus in an inorganic form, exists only in rocks. It is very difficult for living creatures to extract materials from hard rock, so much so that it is simply not energetically profitable to create phosphorus fixing due to the fact that it is too difficult for living creatures to do this.
So how do we still get phosphate and get it to nature?
weathering
Weathering of rocks by water/rain/winds that are carried by the rivers and with it drift to the sea. Currently, we have found no other way than this to source phosphate naturally.
In contrast, the other 5 important atoms can be obtained more easily because they are in liquid or gaseous form, which is something that is much easier for a living creature to put into a cell.
For this simple reason, phosphorus (and other substances such as various metals or non-metals) is always the limiting factor for the development of living things on Earth or in an ecosystem.
This is why, for example, we see blooms of algae when fertilizer is spilled into the sea and suddenly there is a huge bloom of algae that causes ecological destruction.
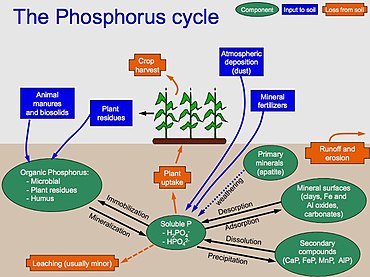
Let me show you a picture:
This is the phosphorus cycle in nature and as you can see - except for rocks (and dust which is technically a solid form, such as meteorites), there is no other source of phosphorus that enters this system, and life has always lived in a lack of it.
They can also be adapted to important evolutionary points, such as the “Cambrian explosion” where there is a theory that says that due to a lot of erosion of minerals and rocks that reached the sea, the amount of materials available to living creatures was increased, which was one of the factors, in addition to the increase in the amount of oxygen in the atmosphere and in the sea, and also in the formation of predator-prey.
Therefore, this is the whole story behind why there is no “phosphorus determination” versus “carbon determination” or “nitrogen determination” (metals are a completely different story, unlike phosphorus, they are not required in large quantities or at all, depending on where, and also about consumption, there is a sufficient amount)
This also includes a political description with fertilizers, for example that 75% of the source of phosphate for fertilizers comes from only one region in Morocco, just because of the fact that I told here, but it is no longer relevant.
TLDR: The problem is not how much phosphate is common, but what is the ratio between consumption by living creatures and the amount that is replenished. Living beings use more phosphate than the amount in which phosphate enters nature.
I remember there is a saying that the lichens that landed early secreted lichen acid, which accelerated the erosion of land rocks, causing a large amount of phosphate to enter the ocean and promote algal blooms.
It is true that it exists, but there is still no exact proof just because it is quite difficult to get proof from that era.
But this could be a factor that probably helped accelerate the weathering. Fungi are also known as this, even today, but in relation to the amount required, it is quite minimal or very limited and delicate and some kind of weathering is required for it to really allow phosphate to enter the circle. Phosphate is not that common in rocks and it requires a fairly large amount of rocks or specific rocks that contain a lot of phosphate for it to really be effective, something that is not common in nature as we currently know.
these points make sense, now that I think of it, extracting useful materials from solids is already an issue I’m dealing with my speculative evolution project, so it makes sense life has more to gain from getting phosphates already available than synthesizing its own from the chemically pure components.
however hhyyrylainen 's explanation have shown me a rather new perspective on ATP, I’ve always heard about it as a form of chemical energy, like that in batteries; but hh makes it seem more like the storage, the battery itself, which reaffirms my fears about this very inneficient process demolising a cell’s energy economy (though these effects on the victim organism open up a few interesting ways to reprupose the process for offensive uses)
Phosphate organelle was suggested a few times before. This suggestion (1) asks for a “phosphate making organelle” but it was rejected because “organelles don’t make phosphate”. This one (2) was more careful with its wording and it asked for a “phosphate organelle” that supplies the cell with phosphate similar to how the ammonia organelle supplies ammonia. I voted for that one.
The ammonia organelle makes NH3 from N2 which is invisible in the game like all the other gasses. So can there be a phosphate source besides the phosphate clouds?
This implies that there is a way to get more phosphate, but it is only achievable by multicellular species. But unicellular species can do that as well. They live around plant roots and secrete acids and chelating molecules to get phosphorus to dissolve into the water*. They accelerate the weathering. “Phosphate is not that common in rocks” but they still evolved the ability to do that.
This can be represented in the game with an organelle that allows cells to make phosphorus chunks release phosphate clouds around them. Ocean patches may not have phosphorus chunks though (3)
“Plant roots should create phosphate” was also suggested (4) but they don’t mention microscopic symbiotes.
A phosphate-fixing organelle would have no extra source of phosphorus to fix to phosphates.
Phosphate-rich floating mineral chunks would be the best way to allow cells to focus on faster growth (along with N-fixing). A new agent could be added to erode P-rich chunks, but I don’t think this is necessary. The mineral chunks could simply emit clouds of phosphate, just as the iron chunks do (although it may be less realistic).
you can eat the iron chunks if you’re big enough though and there is an organelle that speeds up and increases the efficiency of their digestion, for iron though it just happens to also use the iron to make ATP, for a phosphorus fixing organelle it could do the same but turn abiotic phosphorus sources into phosphates
True. Though I feel this is very much in the same realm as siderophores, which were mentioned on the dev forum Organelles / Mutations - #30 by Buckly - Gameplay - Thrive Development Forum. It could add some interesting variety and complexity to the game, but is probably best considered when the game has been more fully fleshed out.