- Basics
- Gas
- Energy
- Reaction speed
- Equilibrium
I’m writing the basics tomorrow.
I’m writing the basics tomorrow.
This smells like GCSE.
I do not like the smell of GCSE.
Smells like stress.
Are you british, sir Biologicah ?
I’m not a big expert in a lot of this stuff. I think the most simple way to understand the scientific method is that it’s a two step process. First you think of a hypothesis and then you try as hard as you can to disprove it. The more you fail to disprove it the stronger it becomes, so it can be upgraded to a Theory or a Law. In Thrive there’s a few place this happens
When you play the game, or any game, you are sort of doing this. You think “maybe I need the yellow cloud to reproduce” and then you test that out, trying to reproduce using it and maybe trying to avoid it to see if you really need it. The scientific method is really just a formal version of trial and error which you use when you learn anything.
When designing the game we make a lot of hypotheses like “the pilus will make combat more fun” and then we go and test that and see if it’s true. So far it’s only a hypothesis but when we add it we’ll start getting data to back up whether it is more fun or not. Just looking at our own experiences isn’t enough, we have to see feedback from a lot of different sources, like these forums, letsplays etc.
One crazy idea I had for the late space stage is to have several different branches science could take. Right now we don’t know what Dark Matter is, for example. So maybe in the game there could be 4 different explanations which lead to different possible technologies. As the player you have to commit scientific resources to unraveling these possibilities. Maybe that’s nuts but is an example of using the scientific method while playing.
Hope that’s helpful 
In fact, by scientific methodology, I meant more about uncertainty and significant figures. Whatever let’s start by the different atomic model through history.
1.1 : Atoms and their representations through history
A. Dalton’s atomic model

It is recommended that you watch this video from at least at 0:40 because the beginning is a spoiler for my text.
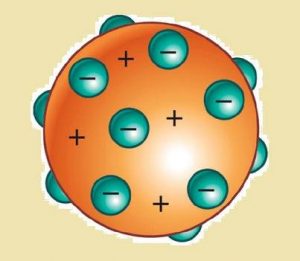

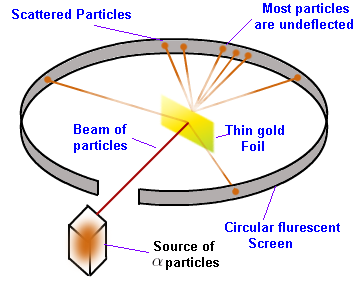
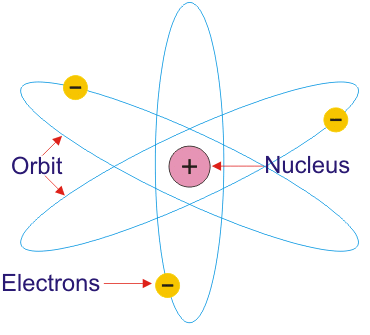
Here is an image illustrating Rutherford’s atomic model.
D. Rutherford and Bohr’s atomic model
E. Simplified atomic model
F. Lewis’ notation
Nice ![]()
I think, as water is a molecule rather than an element, it can be created or destroyed in reactions. If you burn hydrogen then it can bind with the oxygen in the air to make water.
However I think the idea holds true with hydrogen or oxygen, neither of these can be created or destroyed in a chemical reaction.
Edit :
Maybe you should write explicitly about that. I feel like it would be very beneficial for people to know what the difference is.
A mole is a quantity unit equivalent to the Avogadro number, which is equal to $ 6.02214076*(10^23) $ particles.
Can’t do formulas apparently.
Source
I appreciate what you are trying to do but you should really try to fill in the content yourself instead of just mentioning a bunch of people and asking them to fill content just based on a single word.