Abstract according to valence:
The microbial nitrogen-cycling network | Nature Reviews Microbiology
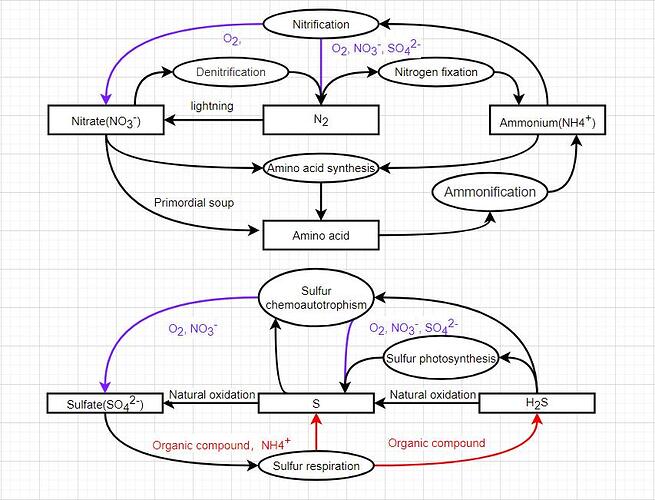
Considering that elemental sulfur is a solid, it should be like current iron.
Sulfates, nitrates, hydrogen sulfide and ammonium serve as environmental compounds.
Sulfates and nitrates should be like current O2 and CO2.
Hydrogen sulfide and ammonium need to be absorbed by cells based on environmental concentration and absorption capacity, and enter storage to work. (Create a process of absorbing (synthesizing) material based on environmental concentration based on the size of cell surface area and membrane type?)
Hydrogen sulfide still has naturally generated clouds in Hydrothermal vent and other special patches.
If ammonia entering the breeding progress bar is regarded as amino acid synthesis, should it be regarded as a controllable process? By coordinating with the process regulation function, cells can reduce consumption by slowing down growth, and photosynthetic bacteria can hibernate at night. This process may also be combined with the environmental tolerance of cells, reducing the rate and efficiency of amino acid synthesis in less adaptable environments.
And the carbon cycle(Not considering methane):