As for NADH, as I was talking about the use of NADPH as an intermediate in autotroph,
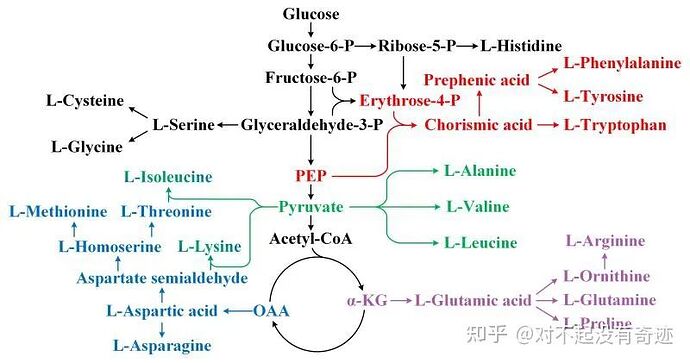
I originally intended to use NADH as an intermediate in glycolysis and aerobic respiration. They should be similar to ATP. The reason why I changed to pyruvate is that I have discovered that pyruvate can synthesize amino acids (I hope to distinguish ammonia and amino acids to achieve ammonia nutrition), but now I have found this:
Consider synthesizing amino acids or using glucose as the substrate directly.
From a gaming perspective, NADH is just an indicator of the intensity of glycolysis, limiting the intensity of aerobic respiration that can proceed next. During stable preparation, NADH should be fully utilized by aerobic respiration. If there is surplus NADH, it should be used for fermentation (it seems that there is no need for a separate organelle for fermentation, perhaps the fermentation type can consider the passive protein system that has not yet been realized?).
For individual storage, if glycogen adds the same amount of storage space to glucose and iron, isn’t that right. Regarding storage, cells enrich substances into semi permeable membranes by active transport and synthesize macromolecular substances, like starch to store glucose separately. The main reason is that there is no need to treat all storage methods as separate compounds unless they have further special uses. For cytoplasm and vacuoles, it is a universal storage device that still works as it does now. Separate storage should appear in the corresponding organelle, which represents the buffer effect of substances.
Compared with the storage of phosphate, the direct storage of ammonia seems more absurd, which can only be said to be a abstraction. Ammonia should be further abstracted as nitrogen to add nitrate. At that time, nitrogen is used to synthesize amino acids and enter the breeding strip. Ammonia (ammonium) and nitrate, respectively, can be absorbed and converted into nitrogen as environmental compounds. It is allowed to add nitrifying bacteria and denitrification bacteria.
edit:
SRB(Sulfate-reducing bacteria) can use pyruvic acid and other organic substances as electron donor to reduce sulfate to hydrogen sulfide, and obtain survival energy from redox reaction. Sulfates are widely present in primitive oceans and hot springs, and can serve as a good anaerobic pathway for glucose metabolism. Should direct oxidation of hydrogen, hydrogen sulfide, and ammonia by sulfate be feasible?
edit:
I just found some information that NADH can serve as a hydrogen donor for nitrogenase nitrogen fixation. In fact, NADPH is also. In addition to ammonia, nitrogenase also has the ability to catalyze the synthesis of hydrogen gas, which is hydrogen fermentation.