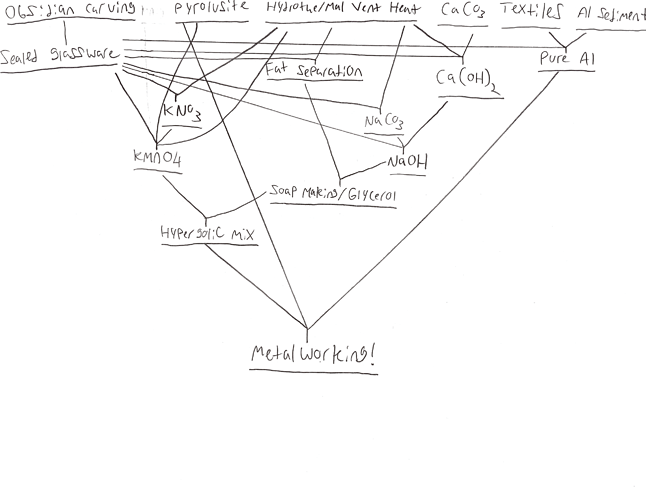
Hhyyrylainen’s Challenge: UC Metalworking Formatted like a Scientific Paper
Abstract
Through the use of obsidian glassware and other simple and inevitable technologies, UCs have all of the precursors and incentive needed to perform metalworking through thermite reactions ignited with hypergolic mixtures of potassium permanganate and glycerol or similarly energetic reactions.
Figure 1 (summary of precursor behaviors / technologies needed for metalworking)
Introduction
I understand that this is a long post, but before you go straight to the comments with quick judgment, watch this video (Slow Mo Molten Thermite in Water - The Slow Mo Guys - YouTube). At 3:00 it clearly shows an underwater thermite reaction producing enough heat to melt aluminum oxide and iron metal. This is conclusive evidence that a reaction exists that can be used for metalworking. There are certainly some caveats like the initial reaction temperature which I will get to, but please give this post a real chance by forgoing your preconceived ideas about underwater civilizations (UCs) and giving this post open minded consideration. In this post I will prove that a thermite reaction could be used to do metalworking and ignited underwater using simple materials found around hydrothermal vents and methane seeps. For how metalworking fits into UC to space, see this (1) forum post. A final note- Like @Steve, I don’t actually care if UCs are added, I just want to convince the UC-metalworking-haters in this forum that UC metalworking (UCM) is possible. Technically, Hhyyrylainen’s Challenge calls for a bachelor’s level thesis, which this is not, but I believe it proves beyond a reasonable doubt that metalworking would be developed by a UC, which should be all that is needed to convince people.
Some Things to Keep in Mind
- Please read my original post for background first (47). In that short post I talk about the theoretical basis on how there are many ways to do metalworking underwater. In this post I focus on a specific hypothetical pathway.
- Innovations don’t have a deadline. Humans developed metalworking relatively quickly in our history, but that doesn’t have to be the case. A species can take 10 million years to develop metalworking and still make it to space. The only real deadlines are mass extinction events that happen on Earth about every 100 million years, which should be magnitudes more time than needed for the process I’m proposing.
- The order of discovering technologies can often be arbitrary. I have read forum posts where people disregard thermite reactions for UCM because they were historically developed after the industrial revolution. Some thought should highlight the fallacy here- just because we developed technologies in this order does not mean that technologies must be developed in this order, especially if it is entirely feasible (which I will show) to do so otherwise.
- View steps as useful for their own sake, not just as a means to UCM. It can be easy to view the steps that I am about to list as solely a means to UCM, and likewise criticize them by saying “why would a UC do this arbitrary step.” However all of the steps listed are useful for their own sake, as I will explain. Keep this in mind while reading.
- Native Aluminum (Un-oxidized aluminum) is found naturally around methane seeps (6). I will explain this more, but I just wanted to state this early so people don’t immediately post “thermite needs native metals” in the comments.
- This is not the only way. I know someone will criticize this by saying “this is way too specific and complicated, the chance of all of these happening is so low.” To the contrary, all of these precursors don’t need to happen, there are countless ways for UCM to work, this is just one possible path that I layed out in extreme detail because my earlier posts were criticized for not being detailed enough. Think about how complicated the steps to human metalworking were. Metalworking is going to be complicated, so please read the whole thing with an open mind.
- I assume we start with a medieval like UC that developed near a hydrothermal vent. Hydrothermal vents (HVs) host high densities of diverse life (2), so they are a good contender for the location of a UC. Most also agree that a UC could reach a medieval state with little challenge (1). I also assume that this all occurs on a planet identical to modern Earth minus humans.
Precursor 1: Pyrolusite Usage
Pyrolusite is a common insoluble mineral consisting mostly of manganese dioxide. It is common all over the world, including around HVs (3), and it is reasonable to assume that members of a UC would collect pyrolusite for its many immediate uses such as a disinfectant, dye, and paint (4). Historically, there is evidence that Neanderthalls did exactly this- sourced pyrolusite for art and fire starting (5).
Precursor 2: Native Aluminum
Like I stated in “Some Things to Keep in Mind,” native aluminum (Al in the 0 oxidation state) is found in the sediments around cold seeps, as well as many other places on the seafloor (6). Aluminum is chemically unique in that it does not rust like other metals, but instead forms a small passivating layer on its surface that protects the rest of it from reacting (7). This allows it to remain shiny even when it is a very fine power, making it a major component of many shiny paints (8). I cannot find pictures of native aluminum sediment, but I assume it would be moderately shiny, especially to a UC that has never seen any other pure metal. Likewise members of a UC may periodically travel to cold seeps to collect native aluminum sediments for use as a shiny pigment as well as various other unique resources cold seeps offer. Historically humans never did this because most native aluminum sediments are found underwater, but humans did collect other aluminum minerals like alum for similar purposes (9). I feel the need to reiterate #6 in “Some Things to Keep in Mind,” there are several other native metals found around HVs and methane seeps, such as native copper (46). Aluminum is not the only way, it is just the one I chose.
Precursor 3: Obsidian Carving
Hopefully the knowledge of early human obsidian usage is widespread enough that I do not have to find a citation for it. Being a strong glass, obsidian is an easy material to construct simple but powerful tools out of, especially spears. Obsidian gravel is often found around HVs (10) making it a great option for UCs to create tools. Obsidian can also be carved by anything harder than itself (40). On Mohs scale of hardness, obsidian is a 5 to 6 (41). There are many materials around HVs that would be hard enough to carve obsidian, including quartz (43) and pyrite (44) (42). I imagine UCs would probably use a mixed method of chipping and carving, as carving alone would take lots of time, but chipping alone wouldn’t allow for fine detail. Obsidian carving would also offer a great alternative to ceramics for a UC. Hollow vessels could be carved and used to hold materials (39).
Precursor 3.5: Sealed Glassware
Most of the materials collected in obsidian vessels would be solids such as muscles and building materials, however there is a key exception- brine from brine pools. At this point in a UC’s history, it is very likely that they have settled around HVs outside of their native habitat because each HV can only provide so much food, and like all human populations, a UC’s population would be growing. In their expansion, a natural option for some members would be to settle around methane seeps because they have already been gathering resources from these seeps such as aluminum sediment. Additionally, the food sources around a methane seep would be broadly similar to those around HVs in that methane seep ecology consists of chemosynthetic bacteria and some symbiotic macro hosts like muscles and tube worms. Like around HVs, the base of the ecosystem depends on nutrients coming from underground flows. Unlike HVs, the dense “brine” that defines methane seeps moves slowly and often forms actual pools called brine pools (45). The system is very sensitive, as the pools are too saline for macrolife to live in them, but macrolife also cannot live too far away because they need the brine for chemosynthesis (see the picture in (45)). UC settlements around methane seeps would understand this, and would be strongly incentivised to develop a kind of “irrigation system” by which brine from the central pool is distributed to muds otherwise too far away to host chemosynthetic life. This may begin with a simple obsidian tool similar to a watering can. Of course a key innovation would be making the tool water tight because any brine collected with it would slowly diffuse otherwise. This could be done with a “cork” made of chitin or cartilage. As time progresses and the need for food further would increase, and UCs would innovate more complex obsidian glassware such as ground glass, which can be air tight if two pieces are cut to fit tightly together (13). By fitting chains of ground obsidian glass together in systems with glass vessels, a UC would be able to make practically any sealed glassware that we could without the need for any glass blowing. This could be used to make complex irrigation systems that would drastically improve the productivity of their food systems. More importantly, UCs could also do complex chemistry with aqueous solutions.
Precursor 4: Take a Deep Breath, Hydrothermal Vent Heat (HVH) Use
Hear me out, I’m not jumping straight to UCM here, I’m just saying that HVH could be used for smaller scale things like cooking food. To address “how would they keep themselves from burning?” I direct readers to this paper (11) “Two-dimensional temperature maps at vent sites have demonstrated order of magnitude thermal changes over centimetere distances.” Furthermore a UC at this stage could easily create tools to allow them to stand further away from the vent. These could be made of obsidian segments or whale ribs, which have been found to be up to 9ft in length (12); plenty of distance to not become burned.
Precursor 5: Textiles
Given any source of fiber available around HVs such as chitin or cellulose, textile production would be identical to the process on land. Both fibers used for human clothes and those available around HVs are insoluble, and since textile assembly does not require any chemical changes the same process as above water could be used. There is also a clear incentive- clothing would offer many of the same benefits to UCs as to humans.
Precursor 6: Aluminum Sediment Refinement
The history of blue pigment has become somewhat pop science but I will restate it anyway. For the majority of human history the only viable blue pigment was the ultramarine in lapis lazuli, however lapis lazuli is rarely pure enough to give a true blue color, so blue pigments were very bad. In the 15th century Cennino d’Andrea Cennini developed a method for separating the ultramarine in lapis lazuli from its impurities (14). The method was very labor intensive- it basically consisted of grinding up lapis lazuli and separating the ultramarine by particle size via sieving it with fine cloth. Although labor intensive, the method gave a very vibrant pigment, skyrocketing the price of ultramarine beyond gold’s (15). A similar situation could occur for the shiny pigments in UCs. Before this point, UCs would have a single, but kind of bad siny pigment- native aluminum sediment. Cennino d’Andrea Cennini’s method could be discovered and used to separate the native aluminum from the impurities with lots of labor. It would be hard but there would certainly be a market for pure native aluminum pigment- the shiniest thing to ever exist in a UC.
Precursor 6: Potassium Nitrate Usage
Potassium Nitrate (KNO3) is a salt which was one of the first major nitrogen fertilizers (16). Like life on the surface, life around HVs also needs nitrogen to grow and would benefit from a nitrogen fertilizer. In fact, nitrate is particularly important for Riftia pachyptila (giant tube worms) that live around HVs because their symbiotic bacteria need it for chemosynthesis (17). This means that a nitrate fertilizer may be even more important for UCs than in surface agriculture! KNO3 is also relatively easy to make, it is produced when animal excrement is mixed with compost in a process known as a nitre bed (18). Of course, this process would have to be done in a sealed obsidian vessel, but it would be 100% possible underwater. Composting is also a very intuitive process that nearly all native humans practice and UCs would too. The fact that much of the modern world doesn’t compost could be an essay on its own. Alternatively the blood of Riftia pachyptila is also very high in nitrate (17). If for some reason you don’t buy the compost method, this blood could be used instead.
Precursor 7: Sodium Carbonate Usage
Sodium carbonate (Na2CO3) is an important food additive for its alkaline properties. In baking, pH is very important for gluten production and Na2CO3 is often used to control this (19). Na2CO3 can be produced by burning plants which grow in saltwater (20). Of course, UCs could not burn plants, but they could extract Na2CO3 through organic separation (21). At this point UCs may actually be baking bread, as saltwater yeast can act similarly to surface yeast (22), so the benefit of Na2CO3 is clear, but before they knew the effect, why would a UC perform an organic distillation on a saltwater plant, then add the product to food? We now enter into the realm of true experimentation. Why did the Tang Dynasty mix powders of charcoal, sulfur, and KNO3 and light it on fire- thereby discovering gunpowder? The answer is that they were literally randomly mixing things together because that is one method of discovering new things (23). Almost every medication before the 19th century was discovered this way, and countless other important technologies. From here on, there is not a great explanation for why a UC would initially try these things, but if there is a clear benefit to doing them (which there is for each of the following, as I explain), they would certainly continue.
Precursor 8: Calcium Carbonate Usage
Calcium carbonate is a very useful material that can easily be obtained by UCs by crushing shells and bones. It can be used as a fertilizer and a pH regulator in baking like Na2CO3.
Precursor 9: Calcium Hydroxide Usage
Under the heat of a hydrothermal vent, calcium carbonate calcifies to calcium oxide (24), then would immediately react with water to form calcium hydroxide (25). Calcium hydroxide can be used as a fungicide (26) or a flocculant (27).
Edit: I forgot that the calcination of calcium oxide requires heating to 900 degrees C, which would be impossible for a UC at this point, however as I discuss in “Precursor 14,” this entire sub pathway to produce glycerol (which calcium hydroxide is used for) is actually unnecessary because ethanol can be used instead. Furthermore, glycerol can also be produced synthetically without the need of the saponification of fats and likewise calcium hydroxide, giving another alternative. Remember “Some Things to Keep in Mind” #6- “This is not the only way.”
Precursor 10: Sodium Hydroxide Usage
Sodium hydroxide can be produced from the reaction of calcium hydroxide with Na2CO3 (28). Sodium hydroxide is a very useful strong base that can be used as a cleaning agent, hair straightener, and to make soap (see soap making).
Precursor 11: Fat Separation
Animal fat can be separated by just heating the fat in water. Since water and fat don’t mix, this will create two layers of liquid which can be physically separated (29). The major reason humans do this is for more complex cooking, but it can also be used for soap making (see soap making).
Precursor 12: Soap Making
Hopefully I don’t have to explain how important soap is. Soap is made through a saponification reaction between fats (triglycerides) and sodium hydroxide. Notably, this process will also create glycerol as a byproduct (30).
Precursor 13: Potassium Permanganate Usage
Potassium Permanganate is on the World Health Organization’s List of Essential Medicines for its disinfecting use (31). It can be created by heating a mixture of manganese dioxide powder with KNO3 under HVH (32).
Precursor 14: Hypergolic Mixture of Potassium Permanganate and Glycerol
When potassium permanganate is mixed with glycerol, the mixture instantly explodes on contact without the need of activation heat in a reaction known as a hypergolic mixture (33). An alternative to glycerol could also be ethanol, which retrospectively would have been a shorter pathway because ethanol can be created by fermenting sugars directly, even in saltwater (22); but glycerol also works. This can be used as an explosive weapon (33) or as a heat source independent of HVH.
Metalworking: Manganese Dioxide / Aluminum Thermite Ignited with Hypergolic Mixture
A hypergolic mixture of potassium permanganate and glycerol is a classic ignition reaction for thermite. Here (34) is a procedure and video that shows iron oxide / aluminum thermite ignited with potassium permanganate and glycerol. Would this work underwater? Yes. Glycerol is not miscible with water (35) and potassium permanganate has low solubility (36). And as previously discussed neither manganese dioxide or aluminum powder are soluble in water. Of course obsidian vessels would also probably be used. Would there be enough heat underwater? Lots of people on the forums say this needs to be proven mathematically “with SI units,” but systems like this are so complex that any mathematical description would need lots of approximations thereby creating lots of error in the result. The only real way to prove this would be to actually try it, however I am convinced this or something similar would actually work. Here’s why- in the video (34) iron oxide / aluminum thermite is used which has an ignition temperature of 3000 degrees C (37), however I am proposing using manganese dioxide / aluminum thermite which has an ignition temperature of 547.8 degrees C (38). So even if the heat transfer between the ignition reaction and the thermite is much lower, the reaction could still proceed as long as any of the thermite momentarily reaches 547.8 degrees C. Still not convinced? It would also be entirely possible for a UC to synthesize the reagents for an even more energetic ignition reaction, I only stopped here because I believe this is enough to ignite thermite.
Citations
- Hhyyrylainen's Challenge: UC to Space Completed
- Biogeography of deep-sea hydrothermal vent faunas - Dive & Discover
- http://www.handbookofmineralogy.org/pdfs/pyrolusite.pdf
- Pyrolusite - Wikipedia
- Selection and Use of Manganese Dioxide by Neanderthals | Scientific Reports
- Redirecting
- ISBN 978-0-08-044495-6
- ISBN 978-0-7503-0688-1
- https://gallica.bnf.fr/ark:/12148/bpt6k2151370/f754.image
- Hydrothermal Vent Community - an overview | ScienceDirect Topics
- Deep-sea hydrothermal vent animals seek cool fluids in a highly variable thermal environment | Nature Communications
- https://www.icrwhale.org/pdf/SC0221-27.pdf
- Glass Syringes for Delivering Drug and Biological Products: Technical Information to Supplement International Organization for Standardization (ISO) Standard 11040-4 | FDA
- Art history, symbolism and legends: The Famous Blue: History of Color # 5- the Middle Ages
- Cennino d’Andrea Cennini’s Ultramarine Blue – Part I – Drypigment.net
- Redirecting
- Redirecting
- Microbial Community Structure of Relict Niter-Beds Previously Used for Saltpeter Production
- https://www.nytimes.com/2010/09/15/dining/15curious.html
- Request Rejected pp. 1198–1199
- 9: Separation, Purification, and Identification of Organic Compounds - Chemistry LibreTexts
- The establishment of a marine focused biorefinery for bioethanol production using seawater and a novel marine yeast strain | Scientific Reports
- ISBN 978-0-691-13597-7
- http://www.solvaypcc.com/safety_environment/0,0,1000044-_EN,00.html
- https://byjus.com/chemistry/calcium-hydroxide/#:~:text=Generally%2C%20calcium%20hydroxide%20is%20prepared,2)%20also%20yields%20this%20compound.
- https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015R0762&from=RO
- http://www.wvdhhr.org/wateroperators/wv_advanced_course/resources/l2u1/l2appendix.pdf
- https://www.scielo.br/j/babt/a/JJkq3N43T8CXVKSKLXcC5xr/?format=pdf&lang=en#:~:text=In%20turn%2C%20green%20liquor%20is,This%20reaction%20is%20slightly%20exothermic.
- ISBN 0-684-81870-1 p. 1069
- Saponification - Chemistry LibreTexts
- World Health Organization model list of essential medicines: 21st list 2019
- https://doi.org/10.1002%2F14356007.a16_123
- https://www.aafs.org/sites/default/files/media/documents/AAFS-2017-B20.pdf
- Thermite Reaction.
- Glycerol | C3H8O3 - PubChem
- Potassium permanganate | KMnO4 - PubChem
- https://www.unitednuclear.com/thermiteinfo.pdf
- ShieldSquare Captcha.
- Thomas Glazed Obsidian Vase - Becki Owens Living
- Hardness - an overview | ScienceDirect Topics.
- pp. 108. ISBN 978-1-57506-042-2.
- Hydrothermal Vent - an overview | ScienceDirect Topics
- Mohs Scale of Hardness
- Pyrite Mineral | Uses and Properties
- NOAA Ocean Explorer: Gulf of Mexico 2002
- http://imc.bas.bg/staff/z_damyanov/Copper.Mar.Geol.pdf
- A Chemist's Perspective: Underwater Metalworking